- Document History
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report to a Moderator
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report to a Moderator
Development of an autonomous platform for CAR T-cells manufacturing
Development of an autonomous platform for CAR T-cells manufacturing
Using LabVIEW and NI Hardware
Company: Aglaris Ltd
Author: Mireia Ruiz, R&D Engineer
NI Products Used: LabVIEW, compactRIO and C Series Modules
Industry: Biotechnology, Research
The Challenge
Create an autonomous system for the complete manufacturing process of CAR T-cells therapies to ensure a controlled and optimised cellular product for a better and more accessible treatment for the patient.
The Solution
Integrating sensors and actuators into a NI hardware platform controlled by LabVIEW to create a data acquisition and processing system that controls the different processes of CAR T-cells manufacturing according to the cellular needs for each patient and ensure an optimised cellular product.
Introduction to CAR-T therapies
For many years cancer treatment has been based on surgery, chemotherapy, radiation therapy or drugs targeting cancer cells. Over the past several years, immunotherapy has emerged as a new way to treat cancer. In particular, CAR-T therapy has rapidly evolved given the remarkable responses produced in some patients [1].
CAR-T therapies are a type of treatment in which a patient’s own T-cells, from its immune system, are genetically modified in the laboratory to incorporate a chimeric antigen receptor (CAR) and recognise and attack cancer cells. These modified cells are injected back to the patient after an expansion process takes place to obtain therapeutic quantities (1x105 to 6x106 cells per kilogram of patient’s body weight depending on clinical trial) [2]. The complete CAR T-cell therapy cycle can be seen in Figure 1.

There are two CAR-T therapies already available in the market to treat specific types of cancer, but these are only offered after the conventional treatments have failed to work for the patient. Thus, when patients undergo CAR-T therapy, it is their last chance for treatment. Also, given their condition at the point and that a quantity of cells needs to be obtained from them, there is usually only one chance for the immunotherapy treatment to work. It is vital to ensure that the manufacturing of the therapy is done as efficiently and optimised as possible to increase the chances of patient survival.
Current methods for CAR T-cells manufacturing include manual steps at some point of the process or lack many sensors to have a completely controlled cellular product at the end of the manufacturing process. Aglaris’ system aims to solve these limitations by creating a completely closed and autonomous system that has sensors’ feedback at each step, adjusts the following stages and controls the operation depending on the performance of the process so far.
The FACER approach
Aglaris has developed the FACER (Fully Automated Cell Expansion Reactor) prototype (see Figure 2) to cover the market limitations in two aspects:
- Complete automation: The process is completely closed, and no human intervention is required.
- Autonomous system: There is constant feedback given by the sensors at all steps of the process to ensure optimal operation and the best quality in the cellular result.
The prototype currently consists of three modules:
- A storage module: cell culture medium and other reagents must be stored at 4ºC and in darkness conditions.
- A culture module: where all the cellular processes take place within a proprietary designed cell culture single use cartridge.
- An interface module: including an industrial PC and a screen to show progress and ask for user input to start operating the system.

A single-use cartridge is inserted in the culture module and used to create the CAR-T therapy corresponding to each patient. Figure 3 shows the connection of the cartridge inside the module at the beginning of the culture.
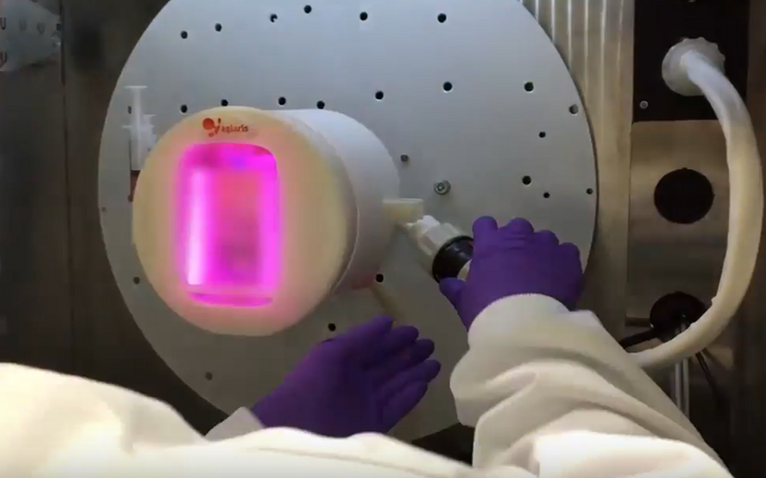
Control Hardware
The main advantages offered by NI hardware were the straightforward data acquisition options, the possibility of integrating different communication protocols in the same controller, the Real-Time processing and the accessibility of data logging. Apart from that, using a compactRIO has been the perfect option for prototyping phases as it has allowed constant changes and no major modifications will be required if we need to adapt the system into a single-board controller in the future to reduce sizing.
The main NI platform used in the system is a cRIO-9067 with an expansion chassis NI-9149. These gives the system a modular approach of 16 slots in total for all the data acquisition and actuation needed throughout the process. The different C Series modules to actuate the components are:
- NI 9201: Voltage analog input module for reading from bubbles, load and pressure sensors
- NI 9226: RTD temperature input module for reading from temperature sensors
- NI 9263: Voltage analog output module for pump actuation
- NI 9421: Voltage Digital Input module for reading from position sensors
- NI 9476: Voltage Digital Output module for pneumatic valves, shaker and peltier actuation
- NI 9870: RS232 Serial Interface module for communication with gas flow controllers, DO, pH, CO2 and metabolites sensors
- EtherCAT: for motor actuation
Software
LabVIEW was chosen as the software used to program our application as it allows direct communication to National Instruments’ hardware and great interfacing with different communication protocols. This made it easy to integrate new components in our prototype by developing the corresponding drivers without the need to spend much time programming new features thanks to LabVIEW built-in functions. Moreover, NI Technical Support and NI partners have been extremely useful throughout the whole developmental process. LabVIEW programming allows tracing processes and data, and correctly identifying different batches of cell products, which is needed to adhere to GMP regulations for manufacturing equipment.
The program constantly acquires data from the different sensors to monitor the parameters in the system in real time. If a given parameter is found to be outside the specified range for a correct cell manufacturing process, the system reacts by activating different routines and controlling actuators so that the parameter can be readjusted, and cells can continue to grow in an optimal environment. In particular, the FACER is able to perform the transduction process to obtain CAR T-cells and the expansion of those cells to get the numbers needed for the corresponding therapy. To do so, the system is able to seed, passage and harvest the cells in a completely automatic way with a constant control of process parameters.
The program includes a user interface that allows following the process at all times with feedback of parameters, cell number, stage and days remaining to complete the operation. Figure 4 shows the user interface created with LabVIEW.
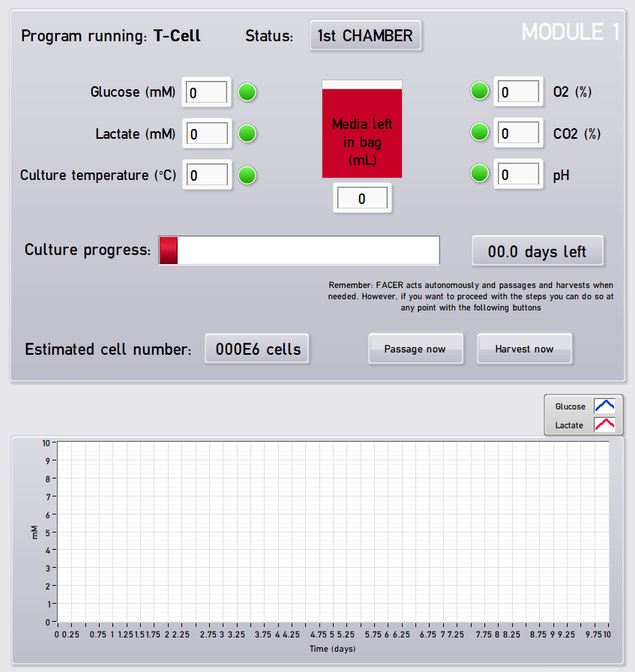
Impact of the developed platform
The FACER system has already been tested with cells coming from real donors and has shown promising results in comparison with current approaches. During a 10-day expansion process, the FACER system obtains twice the number of cells compared with a commercial manual alternative while controlling and maintaining the levels of metabolites and other important culture parameters as seen in Figure 5.
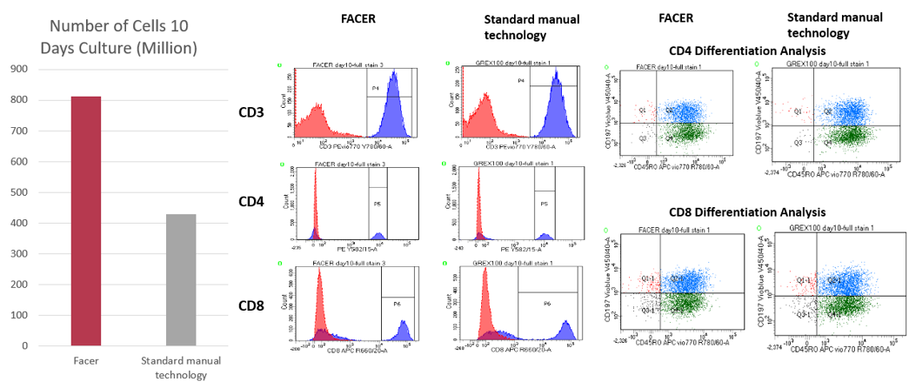
Conclusion
NI platform has been the best option for prototyping our platform and to have it ready for the next steps towards commercialisation of the product. Most importantly, it has allowed us to bring our approach closer to a reality that will hopeful benefit plenty of cancer patients in the following years.
References
[1] National Cancer Institute, 2019, CAR T Cells: Engineering Patients’ Immune Cells to Treat, National Cancer Institute, viewed 29 November 2019. <https://www.cancer.gov/about-cancer/treatment/research/car-t-cells >
[2] Hartmann, J. et al., 2017, Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO molecular medicine, 9(9), 1183–1197. doi:10.15252/emmm.201607485
Author Contact Details
Mireia Ruiz
Aglaris Ltd.
Stevenage Bioscience Catalyst, Gunnels Wood Road
SG1 2FX, Stevenage, United Kingdom
If you want to know more, contact us on: mireia@aglaris.co.uk
